Infection Prevention Orientation Manual
Section 4: Committee Leadership and Membership
Deborah F. Wilson, MS, RN, CIC and Baerbel Merrill, MS, BSN, RN-C, CIC
October 2014
Download a printable PDF Version of this section.
Objectives
At the completion of this section the Infection Preventionist (IP) will:
- Verbalize understanding of the IP’s role in various multidisciplinary committees in the facility
- Verbalize understanding of methods to conduct effective meetings
- Utilize standard formats for recording committee minutes
- Evaluate the effectiveness of committee meetings
Number of hours
- Key Concepts – 5 minutes
- Methods – 3 hours
Overview
The IP possesses a comprehensive skillset aimed at preventing, investigating, and managing the spread of infections within healthcare settings. With an ever-increasing emphasis nationally on prevention, the role of the IP crosses many disciplines in the healthcare setting. The IP is involved in clinical, environmental, and managerial experiences and works closely with personnel from diverse backgrounds.
Key Concepts
- The IP is a member of several multidisciplinary teams.
- In addition to clinical expertise regarding infection prevention and control, the IP has responsibilities in the following areas within the facility: emergency preparedness, safety/biosafety, new product evaluation, construction safety, antimicrobial stewardship, wound care, water safety, environment of care, reusable medical equipment, performance measures, employee health, and nursing policies and procedures.
- The IP acts as a facility liaison with local, county, state, and Federal agencies.
- The IP demonstrates leadership skills during Infection Prevention/Control Committee meetings.
- Effective meetings are structured, have solid objectives, a specific agenda, and a commitment from the leader to involve committee members in planning, preparation, and execution of the meeting.
Methods
In addition to the Infection Prevention/Control Committee, the IP typically is a member of and attends several multidisciplinary committee meetings and workgroups. Committees and workgroups are often facility specific, and can vary throughout time. The following discussion presents the typical committees important to the IP in no particular order. The committees listed are not only found in acute care hospitals but may apply to all healthcare facility types such as long term care, rehabilitation, and ambulatory surgical centers.
Infection Prevention/Control Committee
The Infection Prevention/Control Committee (IP/IC) is typically chaired by the Infectious Disease Physician and/or the IP. Membership in this committee may be defined by a charter (refer to Committee Charter Template in Appendix A) with members appointed by the Chief of Staff and/or the facility Director. The IP/IC membership is tasked with oversight of all infection prevention, and in some facilities, Employee Health activities. Table 1 lists the reports that are often presented to the IP/IC during regular meetings and the corresponding responsible party. (Please see the downloadable PDF version liked at the top of the page to view Table 1) Not all reports or responsible parties are applicable in all healthcare settings. For a list of acronyms used in Table 1, please refer to Appendix G.
Exercise #1
Complete Table 2 as applicable to your facility (Download the printable PDF Version linked at the top of the page to view Table 2). Fill in the names, phone numbers, and e-mail addresses of all members of your facility’s Infection Prevention/Control Committee. Contact each member to introduce yourself. Enlist their support to work together to prevent infections and increase patient/resident safety.
Emergency Preparedness Committee
The Emergency Preparedness Committee (EPC) is tasked with ensuring the facility is prepared to deal with emergency situations occurring both internally and externally. Typically, this committee oversees policies and drills regarding the Emergency Operations Plan, hazardous waste, decontamination, and severe weather. Members of the committee include personnel from all clinical departments, information systems, facilities management, administration, and environmental management. The IP may also participate in emergency preparedness activities outside of the facility at the local, county, and state levels.
The infectious disease impact of a mass casualty incident will vary depending on the nature of the event. Any mass casualty incident that results in overcrowding may lead to a higher incidence of airborne, waterborne, and foodborne disease outbreaks. The role of the IP in the EPC is expanding because of the increased threat of bioterrorism or a pandemic. In situations where the IP is also the Employee Health Nurse, the IP would be responsible to monitor the health of the response personnel if decontamination in a Level C is involved. Level C protection must be utilized when a hazardous/chemical or radiological spill occurs. Level C training is required for anyone participating in a Level C operation. Training is offered through Kipp Sanders, Kipp@ready-response-training.com or by contacting Martin Roark, Wyoming Homeland Security, Training Program, Staff Instructors at 307-358-1920. Please read the Wyoming Infection Prevention Orientation Manual (WY IPOM) Section #15, Emergency Preparedness for more details on this committee and the role of the IP in emergency preparedness/management.
Safety Committee
The Safety Committee is tasked with providing a programmatic framework to reduce the risks for patients, staff, and visitors to the facility. The program includes activities that are designed to evaluate risks that may adversely affect the life or health of patients, residents, visitors, and those who work in the organization, and to integrate safe practices into the daily operations of the facility. The goal is to prevent incidents and injuries, including occupational disease and exposure to hazardous materials. Committee members may include representatives from the Chief of Operations, Safety and Occupational Health, infection control, radiology, security police, facilities management, safe patient handling, environmental management services, emergency management, human resources, purchasing/logistics, and biomedical engineering.
Clinical Product Review Committee
The Clinical Product Review Committee is tasked with ensuring standardization of products used in the facility. This committee is responsible for ensuring that the quality of products used in the facility are consistent with economical purchasing and distribution, and meet the needs of the facility, as well as the patient/resident and clients. Committee membership may include representatives from purchasing/logistics, inpatient and long-term care units, biomedical engineering, Chief of Staff/Medicine, surgical services, radiology, infection prevention, sterile processing, quality management, laboratory, pharmacy, prosthetics, and other clinical specialties as needed.
The IP’s participation in this committee is important because new, safer products are often presented to the members during meetings. The IP’s participation on this committee is to review the products recommended for safe handling, especially if the products are chemicals, cleaning agents, and/ or disinfectants, and what kind of training will be required. The U.S. Occupational Safety and Health Administration (OSHA) requires every facility to implement engineering and work practice controls to eliminate or minimize employee exposure to blood-borne pathogens.1 Engineering controls include sharps disposal containers, self-sheathing needles, and safer medical devices that reduce or remove the hazard of blood-borne pathogen exposure. Additionally, OSHA requires that these engineering controls be “examined and maintained or replaced on a regular schedule to ensure their effectiveness,” 1 reflecting the changes in technology that reduce or eliminate the risk of exposure to blood-borne pathogens.
Construction Safety Committee
The Construction Safety Committee is responsible for establishing policies for maintaining a healthy environment of care for patients and visitors, and a safe and healthy worksite for employees and contractors as related to construction activities. The implementation of the construction safety program is intended to reduce the potential for injuries and illness to patients, employees, and visitors from unsafe construction activities conducted by contractors and facility employees. The construction safety program also reduces the potential for facility liability that could result from construction accidents. Committee membership may include representatives from facilities management, environmental services, security, maintenance, safety, industrial hygiene, contracting, infection control, patient safety, security police, and construction project managers.
The role of the IP related to membership on the construction safety committee is to describe measures for the identification and protection of “at risk” patients, control of exposure, and prevention of the spread of infection before, during, and after construction, demolition, renovation, and repair projects at the facility. The IP should attend all architectural construction planning sessions and construction progress meetings and will guide the designers and contractors regarding adherence to infection control procedures and regulatory requirements. The IP collaborates with the construction project manager to develop the infection control risk assessment (ICRA) and subsequent Infection Control Permit for Construction.
Pharmacy, Therapeutics, and Nutrition/Antimicrobial Stewardship
The Pharmacy, Therapeutics, and Nutrition/Antimicrobial Stewardship committee is responsible for oversight of inpatient and outpatient “prescriptions” for patients receiving medical care at the facility. The term “prescriptions” includes any product designated by the U.S. Food and Drug Administration as a drug, including prescription medicines, vaccines, parenteral nutrition, blood derivatives, and intravenous solutions, to name a few. This committee is responsible for reviewing changes in the facility formulary and the facility antibiogram. Of specific interest to the IP regarding the formulary includes changes in antimicrobial, antiviral, and antifungal agents. Additionally, the IP may participate in an antimicrobial stewardship program with other facilities in the same community or region. Please read the WY IPOM Section #12, Antimicrobial Stewardship for more details.
Wound Care Committee
The Wound Care Committee is responsible for reviewing all cases of healthcare-associated pressure ulcers. Additionally, the committee reviews new products and makes recommendations for treatment of difficult to manage wounds. The IP may hold a dual role in infection prevention and wound care. This committee usually is comprised of a physical therapist, nurse (a certified wound care nurse is preferred), and pharmacy staff. Additionally, it would be preferable to have a general physician as a member of the committee as opposed to a surgeon.
Water Quality Safety Committee
The Water Quality Safety Committee provides oversight regarding water quality for the facility by monitoring water testing results and providing mitigation recommendations. Of particular importance in recent years is the presence of Legionella pneumophilia. Legionella species live in a variety of freshwater habitats but are often difficult to isolate. The Centers for Disease Control and Prevention (CDC) created a program for laboratories to test their Legionella isolation techniques against standardized samples. This program is called the ELITE Program, and the certified members list is available on the CDC website.2 The role of the IP as a member of this committee is to be aware of the contaminants isolated from the facility’s water system and ensure mitigation occurs when necessary. This committee can often be rolled into the functions of the IP committee. Additionally, the IP would conduct clinical surveillance for diseases related to the water-borne contaminant. For additional information on the basics of surveillance, please read the WY IPOM Section #5, Surveillance.
Environment of Care (EOC) Rounds
A multidisciplinary group of employees conducts routine rounds to assess the condition of the facility, grounds, and equipment. Each member of the EOC rounds team uses a checklist appropriate to his/her discipline to gather data. The data collected during these rounds provides the Safety Committee with information to make the best decisions regarding safety concerns. The IP as a member of this team would observe compliance with processes such as hand hygiene, isolation compliance, and sharps safety. An additional responsibility of the IP as a member of this team includes reviewing issues that may cause unsafe practices. It is recommended that the new IP develop a checklist outlining the items which will be surveyed by the EOC rounds.
Reusable Medical Equipment (RME) Committee
The multidisciplinary RME Committee membership includes the RME Coordinator, Sterile Processing Supervisor, IP, Surgical Services representative, Nursing Service representative, Patient Safety Manager, a Bio-Medical Engineering representative, and any assigned ad hoc members. The RME Committee has oversight responsibility for reusable medical equipment and defines procedures for the design and implementation of a systematic approach for the set-up, proper use, reprocessing, and maintenance of all RME used in the facility. The RME Committee also has oversight responsibilities of RME standard operating procedures (SOP), ensuring that the procedures remain current and in compliance with the specifications of the written manufacturers’ instructions. The RME Committee is responsible for ensuring a process is in place to systematically retire and replace older equipment, as well as that from different manufacturers, so as to assure continuity of care, SOP training, and competencies.
Performance Measures and Monitors Committee
This committee is also often called the Quality Assurance and Performance Improvement Committee. For more details on quality assurance and performance improvement in general, please read the WY IPOM Section #16, Quality Assurance and Performance Improvement. During meetings of this particular committee, leaders or champions in various disciplines in the facility present items that have not met a selected benchmark. The leaders/champions are responsible for data management and analyses, which are critical factors in each of the quality management system components. Data management and analysis includes, but is not limited to, gathering and critically analyzing data relevant to quality and safety, ensuring data is valid and reliable, comparing the data analysis results with established goals or internal or external benchmarks, identifying specific opportunities for improvement, and implementing and evaluating actions until problems are resolved or improvements are achieved. During the committee meeting, members listen to the reports, request clarification, and make suggestions for improving processes.
Occupational Injury Review Board
The Occupational Injury Review Board acts as a fact-finding committee to review selected occupational exposures and traumatic injuries. The board then determines, by use of reports, witnesses, on-site inspections, and any other pertinent data, the cause(s) of the incidents and corrective actions to be taken to prevent a recurrence. The ARB also educates employees and managers to assist in preventing future incidents and in providing a safe work environment. The ARB has oversight responsibility for incident investigations conducted by supervisors. The ARB reviews all incidents reportable to the Department of Labor through workers’ compensation (i.e., those involving deaths, lost time, and medical expenses). In addition, any non-reportable incidents such as multiple injuries to the same employee, all needle sticks, over exertion/back injuries, and any noticeable trends are reviewed. In many facilities this board is part of the Human Resources Department.
Nursing Policy and Procedure Committee/Practice Council
The Nursing Policy and Procedure Committee/Practice Council members are responsible for policy development and review as it relates to patient care practices and procedures. This committee is also responsible for reviewing nursing competency assessment data. As a member of this Council, the IP participates in policy development and review as the infection control subject matter expert.
Communicable Disease Committee at the County Health Department
The IP acts as the liaison between the facility and the County Health Department. In this role, the IP understands the communicable disease issues that impact the local healthcare facilities allowing the facility to take a proactive stance regarding communicable disease transmission prevention. The focus of this committee is to enhance communication between the healthcare facility and the local public health department.
Exercise #2
Complete Table 3. (Please download the printable PDF version that is linked at the top of the page to view Table 3). For each committee listed, fill in the usual meeting date/time and location and the name of the committee chair. Not all facilities have all of the listed committees or they may not be currently active; if this is the case in your facility, enter N/A in the corresponding row.
Documentation and Reporting
Running Effective Meetings
The IP assumes a leadership role during the Infection Prevention/Control meetings. To run an effective meeting, the IP needs to consider the meeting’s objective, be respectful of participants’ time, and follow a sensible process.
The Meeting’s Objective. The IP should be aware of the intended outcome of the meeting. Possible outcomes include 1) a decision, 2) idea generation, 3) status reporting, 4) communication, and 5) planning. To focus on the objective, the IP could complete this sentence: “At the close of the meeting, I want the group to….”3 When the IP understands the objective(s), the agenda can be developed.
Use Time Wisely. It is important to adhere to activities leading to accomplishment of the objective. The agenda is an important document for the IP to refer to during the meeting to keep the meeting on topic and on time. To prepare the agenda, the IP should know 1) what absolutely should be covered, 2) what needs to be accomplished, 3) who needs to attend, 4) sequence of topics, 5) time allotment for each topic, and 6) when and where the meeting will take place (please refer to the Sample Meeting Agenda Template in Appendix B). Once the IP establishes topics to be covered and for how long, participants should be notified about their expected role and what they need to do to prepare for the meeting. A great way to increase involvement during the meeting is to pre-assign a particular topic of discussion to various committee members.
The time allotted for the meeting must be respected by all meeting participants. The IP should start the meeting on time and should not recap discussions for latecomers. The IP may acknowledge latecomers with a nod, or not at all. If work can be accomplished prior to the meeting, the IP can circulate reports to committee members beforehand. The agenda is a useful tool for managing time during the meeting. If the IP notices that the discussion on a particular topic is near the end of the allotted time, the IP may choose to acknowledge the short amount of time left for the topic, push for a decision, defer the discussion for the next meeting, or assign the topic to a subcommittee or workgroup. The “parking lot” is a valuable tool to keep track of topics people want to discuss but do not have time during the current meeting. Items not addressed at the meeting will be added to the agenda for the next meeting and as such, items considered in the “parking lot” are not deleted unless the issue has been resolved, addressed to the satisfaction of the members, and/or the issue does not exist anymore.
Follow a Sensible Process. Prior to the meeting, the IP should circulate the agenda and request feedback regarding topic choice and time allotments. The IP should notify all people expected to submit reports (refer to Sample Meeting Schedule of Reports in Appendix C) prior to the scheduled meeting. During the meeting the IP should consider the following points:
- Do not allow people to dominate the discussion; ask others for their ideas.
- Quickly summarize each agenda topic after the discussion and request confirmation of accuracy from the committee members.
- Make note of topics that will require further discussion.
- Pay attention to committee members’ body language and adjust as necessary.
- If the discussion strays from the topic, redirect back to the topic.
- Document all assignments that were generated during the meeting. Specify who is to do what by when.
- At the conclusion of the meeting, summarize the next steps.
Preparing Meeting Minutes
Minutes of the meeting describe what was discussed and decided during the meeting. This document provides a permanent, tangible record of the meeting for its participants and a source of information for members who were unable to attend. Additionally, minutes can act as a reference point when the meeting’s outcomes impact other collaborative activities or projects within the facility. Further, minutes are useful to notify or remind individuals of the tasks assigned to them and the timelines. Infection Prevention/Control Committee minutes are reviewed by surveyors from external reviewers.
Meeting minutes should include the following:
- List of attendees: record those in attendance, those absent, and those excused. In addition to documentation of a quorum, this record will allow participation to be reviewed at the end of the year (please refer to the Sample Meeting Attendance Roster in Appendix D).
- Approval of the previous minutes: document any additions or changes to the previous minutes and who requested those changes.
- Old business: include recurrent items that are covered on a monthly or scheduled basis. Items should have a title or tracking number in order to follow-up until the item is closed. Examples of old business items include reports of educational activities, recurring reports, performance improvement measures, and focus review findings (e.g., root cause analysis findings).
- New business: include new facility policies/procedures, performance measure updates, information from other facility committees, and new issues. Issues documented under “new business” can be listed under old business if unresolved after the first meeting.
- Adjournment: include the time the meeting was adjourned, and the date/time/location of the next meeting.
Common-Sense Rules for Writing Minutes: When writing minutes, avoid negative or slanderous information, gossip, derogatory remarks, or verbatim transcription of angry, sarcastic, or joking comments. Record information accurately and truthfully and never falsify minutes, even if asked to do so. Close open items only when there is a resolution, and be able to track the items through the minutes (tell the story). Do not include patient names or identifiers in minutes because, except for a few special situations, most minutes are not confidential documents. Always identify people/speakers with either their whole name (e.g. John Jones, RN) or at least the surname and title. Whenever possible, identify by position name as well, e.g. ICU Director vs. Ingeborg Grunner. Table 4 provides a quick reference for what the meeting minutes should and should not contain.
Meeting Minutes Should Have
- Concise descriptions
- Adequate information
- Actions assigned
- Follow-up to outstanding action items
- Committee title, meeting date, attendance, signatures
- Trended data with analysis
Meeting Minutes Should Not Have
- Joe said and Jane said…
- One line for each item
- No action items at all
- Cliffhangers without resolution
- Last month’s information mixed with this month’s information and agenda and…
- One month’s worth of data or no explanation of data
Evaluation of Committee Effectiveness: External reviewers such as The Joint Commission, Det Norske Veritas (DNV), or the Centers for Medicare and Medicaid Services evaluate committee effectiveness by examining the meeting minutes. The review typically focuses on four main elements of the minutes:
- Utilization of a standardized format.
- Inclusion of the analysis of aggregated data over time.
- The quality of systematic analysis of the data and issues – the minutes should guide the reader through the process of data analysis leading to the development of conclusions. The IP must ensure that the data analysis is understandable to anyone who reads the minutes.
- Ongoing follow-up until the item is closed – it is essential to identify actions stemming from the discussions and determine due dates and persons responsible for the actions. The reader should be able to follow a topic from its inception to its closure by following the meeting minutes. Refer to Figure 1.
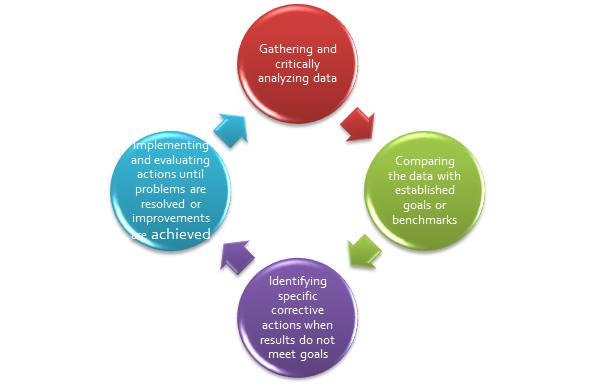
Figure 1. Closing the Loop. Provided by D.F. Wilson, Cheyenne, VA, Medical Center, The Importance and How-To of Awesome Meetings. April 24, 2012.
Resources
Helpful Contacts (in WY or US)
- Cody Loveland, MPH, Infectious Disease Surveillance Epidemiologist and HAI Prevention Coordinator, Wyoming Department of Health, 307-777-8634, cody.loveland@wyo.gov
- Deborah F. Wilson, MS, RN, CIC, Infection Preventionist/MDRO Prevention Coordinator, Cheyenne VA Medical Center, Deborah.wilson5@va.gov, 307-778-7550, ext. 7091
- Baerbel Merrill, MS, BSN, CIC, Infection Preventionist, baerbel.merrill@ccmh.net, 307-698-3942
Related Websites/Organizations
- Wyoming Department of Health, Infectious Disease Epidemiology Unit, Healthcare-Associated Infection Prevention: https://health.wyo.gov/publichealth/infectious-disease-epidemiology-unit/heathcare-associated-infections/
- Mountain-Pacific Quality Health – Wyoming: mpqhf.com/wyoming/index.php
References
- 29 CFR, 1910.1030, Bloodborne Pathogens. United States Department of Labor, Occupational Safety & Health Administration website. Available at: osha.gov/pls/oshaweb/owadisp.show_document?p_table=STANDARDS&p_id=10051. Accessed February 13, 2014.
- Legionella (Legionnaires’ Disease and Pontiac Fever). Centers for Disease Control website. Available at: cdc.gov/legionella/index.html. Accessed February 18, 2014.
- Mind Tools: Running Effective Meetings. Available at: mindtools.com/CommSkll/RunningMeetings.htm. Accessed February 12, 2014.
Appendices
Please download the printable PDF version of this section (linked at the top of this page) to view the following appendices:
Appendix A: Committee Charter Template
Appendix B: Sample Meeting Agenda Template
Appendix C: Sample Meeting Schedule of Reports
Appendix D: Sample Meeting Attendance Roster
Appendix E: Sample Meeting Minutes Template
Appendix F: Sample Meeting Issue Log
Appendix G: Commonly Used Acronyms and Abbreviations

WIPAG welcomes your comments and feedback on these sections.
For comments or inquiries, please contact:
Cody Loveland, MPH, Healthcare-Associated Infection (HAI) Prevention Coordinator
Infectious Disease Epidemiology Unit,
Public Health Sciences Section, Public Health Division
Wyoming Department of Health
6101 Yellowstone Road, Suite #510
Cheyenne, WY 82002
Tel: 307-777-8634 Fax: 307-777-5573
Email: cody.loveland@wyo.gov

