Infection Prevention Orientation Manual
Section 8: Medical Instrument & Device Reprocessing
Baerbel Merrill, MS, BSN, CIC and Deborah F. Wilson, MS, RN, CIC
October, 2014
Download a printable PDF Version of this section.
Objectives
Upon completion of this section the Infection Preventionist (IP) will be able to:
- Demonstrate basic knowledge of cleaning, disinfection, and sterilization of reusable medical equipment (RME) / devices.
- Outline the key points for workflow, transportation, and storage of medical equipment/devices for sterile processing (SP), operating rooms (OR) including c-section suites, interventional radiology, and endoscopy departments.
- Describe the Spaulding Classification System (SCS) for reusable medical equipment and give examples for each category.
- Applies Association of Perioperative Registered Nurses (AORN) and Association for Advancement of Medical Instrumentation (AAMI) standards in the reprocessing and management of medical equipment and devices.
- Describe requirements for air handling systems.
Number of hours
- Key Concepts – 8 hours
- Methods – 8 hours
Required Readings
- Grota P, Allen V, Boston KM, et al, eds. APIC Text of Infection Control & Epidemiolo 4th Edition. Washington, D.C.: Association for Professionals in Infection Control and Epidemiology, Inc.; 2014.
- Chapter 31, Cleaning, Disinfection, and Sterilization, by WA Rutala and DJ Weber
- Chapter 32, Reprocessing Single-use Devices, by R Curchoe
- Chapter 106, Sterile Processing, by J Jefferson and M Young
- Chapter 111, Laundry, Patient Linens, Textiles, and Uniforms, by C McLay
- Chapter 112, Maintenance and Engineering, by SD Cutter
- Chapter 113 Waste Management, by WJ Pate
- Chapter 114, Heating, Ventilation, and Air Conditioning, by J Bartley and R N Olmted
- Rutala W, Weber DJ, et al. Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. Available at: cdc.gov/hicpac/pdf/guidelines/Disinfection_Nov_2008.pdf
Overview
Invasive procedures involving contact of a surgical instrument or medical device with the patient’s mucous membranes or sterile tissues must be safely processed. The goals of safe processing of medical equipment/devices and surgical instruments include:
- Prevent the transmission of microorganism to patients and personnel
- Minimize damage to medical equipment/devices from foreign substances (e.g., blood, body fluid, saline, and medications) or inappropriate handling, transportation or storage.
The IP must know how to clean, disinfect, and sterilize the equipment according to the instrument manufacturers’ instructions. The IP consults with the operating room unit manager to assure best practices and evidence based procedures including monitoring and evaluating practices, and monitor patient outcomes.
Key Concepts
Spaulding Classification System for Reusable Medical Equipment
The SCS for RME, introduced in 1968 by Dr. E. H. Spaulding, divides medical devices into categories based on the risk of infection involved with their use. The SCS is endorsed by the Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) as a means of determining the degree of disinfection or sterilization required for medical devices. The SCS recognizes three categories of medical devices with associated levels of required disinfection:
- Critical: a device that enters the vascular system or normally sterile tissues. Critical devices should be sterilized, which is defined as the destruction of all microbial life.
- Semi-critical: a device normally does not penetrate sterile tissues and comes into contact with intact mucous membranes. Semi-critical devices should be disinfected by high-level methods. High level disinfection is defined as the destruction of all vegetative microorganisms, mycobacterium, small or non-lipid viruses, medium or lipid viruses, fungal spores and some bacterial spores.
- Noncritical: a device that does not touch the patient or comes in contact with intact skin. These devices should be cleaned by low-level disinfection.
Exercise #1
Decontamination / Cleaning
Decontamination protects workers who come into contact with microorganisms on medical devices. Decontamination uses physical or chemical methods to remove, inactivate, or destroy pathogens on the equipment/device to render it safe. The decontamination life cycle (Figure 1) represents the extent to which decontamination affects the entire health care facility. Effective decontamination requires the attainment of standards through all stages of the life cycle. Failure to achieve decontamination standards will result in possible transmission of infectious organisms.
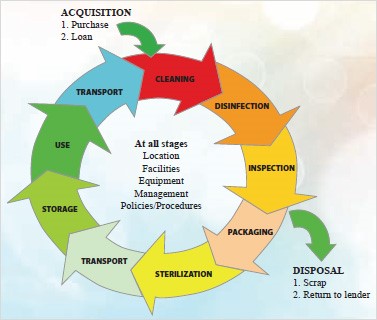
Figure 1. Decontamination Life Cycle.1
An effective instrument reprocessing program includes the following critical elements:
- Pre-treatment: blood, mucus, or other biohazardous materials may cause damage to an instrument if allowed to dry. Enzymatic solutions (react and split the molecules of proteinaceous residues) with a neutral pH are preferred to pre-soak instruments. Never use a disinfectant for this purpose. Instruments should not be soaked overnight or over a weekend due to possible damage to the finish on the instrument.
- Rinsing: rinse with warm distilled water or demineralized water as tap water may leave spots on the instruments.
- Cleaning: remove visible soil such as organic and inorganic material. Clean manually or mechanically using water with detergents or enzymatic products. An instrument that is not completely clean may not be successfully sterilized because the sterilant cannot effectively penetrate the bioburden layer. Light to moderate soils can be treated with neutral detergents; however, alkaline detergents are necessary to lift the debris in heavier soils.
- Rinse and Dry: to avoid rust, instruments must be rinsed and dried using lint-free towels, not treated with bleach or harmful chemicals. Use of high pH (9-13) detergents and/or bleach result in improperly rinsed towels for drying instruments.2
- Lubrication: proper lubrication maintains longevity of the instrument and helps to prevent spotting, staining, corrosion, and sticking/tightness. Use water soluble (non-silicone-based) lubricants on instruments.
- Packaging/wrapping: check instruments for functionality prior to wrapping. Dissimilar metals must be separated from each other. All instruments must be sterilized in the open/unlocked position to allow the sterilant full access to all surfaces.
- Disinfection / Sterilization: adhere to the manufacturers’ instructions for disinfection or sterilization. Failure to follow the proper disinfection/sterilization processes may damage the instrument. The following sections describe the basics of disinfection and sterilization.
Exercise #2
Use the information contained in this chapter and the required readings to define key terms related to the removal of debris and give an example: Cleaning, detergents, enzymatic cleaner.
Exercise #3
Use the information contained in this chapter and the required readings to answer the following questions regarding the cleaning process:
- Why is cleaning the first step of reprocessing?
- Describe the steps involved in the cleaning of medical devices.
Disinfection
There are three distinct levels of disinfection and these include:
Low Level Disinfection: Low level disinfection destroys all vegetative bacteria (except tubercle bacilli and bacterial spores), lipid viruses, some non-lipid viruses, and some fungi.
High Level Disinfection (HLD): High level disinfection uses a chemical or physical agent or process that destroys some bacterial spores when used in sufficient concentration, temperature, and other appropriate conditions. HLD is effective against vegetative bacteria, fungi, and viruses.
Sterilization: Sterilization is a process that completely eliminates all forms of microbial life through physical or chemical processes. Sterilization processes includes steam under pressure, dry heat (ethylene oxide, hydrogen peroxide, gas plasma), and chemical sterilants (liquid immersion).
Exercise #4
Use the information contained in this chapter and the required readings to define and give an example of the following key terms: Low level disinfection, High level disinfection, Sterilization.
Exercise #5
Use the information contained in this chapter and the required readings to answer following the questions:
- Why is it important to classify the device before determining the disinfection method?
- What is the difference between cleaning and disinfection?
Exercise #6
Take a tour of your facility and track down information requested in Tables 6 and 7 (Please see the printable PDF version of this section, linked at the top of the page, to view tables 6 and 7).
Chemical Agents Used as disinfectants: The Environmental Protection Agency (EPA) regulates disinfectants and sterilants used on environmental surfaces and the FDA regulates chemicals used on critical and semi-critical medical devices. A list of products registered with the EPA and labeled for use as sterilants or tuberculocides is available through the EPA’s website at www.epa.gov/oppad001/chemregindex.htm. Federal regulations require that these chemicals be registered before sale or distribution. The manufacturer must submit specific data about the safety and effectiveness of each product to obtain registration. Federal regulations require users explicitly follow the product label directions. Failure to follow the specified use-dilution, contact time, or method of application is considered product misuse which may render the user subject to enforcement action. One of the most common misuses of chemical agents used as disinfectants is the lack of sufficient wet contact time.
The following section includes an overview of the characteristics each chemical and provides information for the user.
Alcohol. Ethyl alcohol (70%) and isopropyl alcohol (20%) are both rapidly bactericidal against vegetative forms of bacteria, tuberculocidal, fungicidal, and virucidal, but not sporicidal. It is thought that alcohol exerts antimicrobial actions by the denaturation of proteins. Generally, alcohols are not recommended for sterilization because they lack sporicidal action and they cannot penetrate protein-rich materials. Additionally, alcohols may damage equipment, are flammable, and evaporate rapidly.
Chlorine and Chlorine Compounds. Hypochlorites are the most commonly used chlorine disinfectants in the healthcare setting and are available as a liquid (e.g., sodium hypochlorite) or solid (e.g., calcium hypochlorite). In water, sodium hypochlorite undergoes a process called dissociation, resulting in a separation of the sodium ion and the hypochlorite ion. Depending on the pH of the solution, the hypochlorite ion may or may not form hypochlorous acid. Free available chlorine (FAC), the hypochlorite ion and hypochlorous acid, determines the sanitizing ability of a bleach solution. Test strips suitable for testing bleach should test for FAC over all possible concentration ranges. The problem is that most test strips are able to test for chlorine within a fairly narrow range.
Hypochlorites are broad spectrum in nature, do not leave toxic residues, are not affected by hard water and are able to remove dried or fixed organisms/biofilms from surfaces. Sodium hypochlorite may produce irritation to mucous membranes and may cause damage to surfaces.
Glutaraldehyde. The biocidal activity of glutaraldehyde results from alteration of Ribonucleic Acid (RNA), Deoxyribonucleic Acid (DNA), and protein synthesis. Glutaraldehyde is most commonly used as a high-level disinfectant for equipment such as endoscopes, hemodialysis systems, anesthesia and respiratory therapy equipment. It is not corrosive to metal and does not damage lensed instruments, rubber or plastics. However, it is very toxic and expensive to use for non-critical surfaces. Healthcare worker exposure to glutaraldehyde should be monitored to ensure safety in the work environment.
Glutaraldehyde concentration declines after a few days of use in an automatic endoscope washer when instruments are not thoroughly dried and water is carried with the instrument. Therefore it is imperative that the concentration be monitored with the use of chemical test strips. Test strip bottles should be dated when opened and used for the period of time indicated. Results of the concentration monitoring should be documented.
Hydrogen Peroxide. This chemical works by disrupting membrane lipids, DNA, and other essential cell components. Hydrogen peroxide is active against bacteria, yeasts, fungi, viruses and spores. It is useful for disinfecting soft contact lenses, tonometer biprisms, ventilators, fabrics and endoscopes. If inadequately rinsed, hydrogen peroxide use may result in a chemical irritation of mucous membranes or even corneal damage. The dilution of hydrogen peroxide must be monitored by regularly testing the minimum effective concentration.
Ortho-phthalaldehyde (OPA). OPA works by interacting with amino acids, proteins, and microorganisms. The lipophilic nature of OPA assists its uptake through the outer layers of mycobacteria and gram-negative bacteria. OPA has several advantages over glutaraldehyde, including excellent stability over a wide range of pH values, lack of irritation to mucous membranes, barely perceptible odor, no requirement for exposure monitoring, and excellent material compatibility. OPA must be disposed of carefully and must be neutralized prior to disposal through the sanitary sewer system.
As with glutaraldehyde, the concentration in automatic washers declines after a few days. The minimum effective concentration must be reached during the reuse process, so the use of a test strip is recommended before each reprocessing cycle.
Peracetic Acid. This chemical sterilant is characterized by rapid action against all microorganisms by denaturing proteins and disrupting cell walls. It lacks the harmful decomposition products found in other chemical disinfectants, enhances removal of organic material, and leaves no residue. However, it is corrosive to many metals and may become unstable when diluted. In an automated machine, it is used to chemically sterilize medical, surgical, and dental instruments. Test strips are used to test low level/residual concentrations of peracetic acid.
Peracetic Acid and Hydrogen Peroxide. This sterilant has bactericidal properties due to disruption of cell walls. It is commonly used for disinfecting hemodialyzers. Test strips are used to instantly determine if the chemical concentration is within an effective range.
Phenolics. In high concentrations, this chemical poisons the protoplasm by penetrating and disrupting the cell wall and precipating the cell proteins. In low concentrations, bacterial death occurs by inactivation of essential enzyme systems and leakage of essential metabolites from the cell wall. Phenolics are absorbed by porous materials and can be irritating to tissue. They are used on environmental surfaces and noncritical medical devices. Phenolics are not cleared by the FDA to use as high-level disinfectants on semi-critical items. They are not to be used in nurseries or kitchen where a possibility for contact with food may occur.
Quaternary Ammonium Compounds. These chemical disinfectants exert bactericidal action by inactivating energy-producing enzymes, denaturing essential cell proteins, and disrupting the cell membrane. Quaternaries are generally fungicidal, bactericidal, and virucidal but are not sporicidal or tuberculocidal. The quaternaries are generally used for environmental sanitation of noncritical surfaces and noncritical medical equipment.
Exercise #7
Sterilization
Sterilization results in the destruction of all microorganisms on the surface of an item or in a fluid to prevent disease transmission. Reusable Medical Equipment (RME) having contact with sterile body tissues or fluids is considered critical and must be sterilized by one of the processes described below.
Steam Sterilization. This process involves the use of moist heat in the form of saturated steam under pressure. Moist heat destroys microorganisms by the irreversible coagulation and denaturation of enzymes and structural proteins. Steam sterilization’s advantages are that it is nontoxic, inexpensive, rapidly microbicidal, sporicidal, and rapidly heats and penetrates fabrics. Its disadvantages include damage to some materials.
The four parameters of steam sterilization include: 1) steam, 2) pressure, 3) temperature, and 4) time. The best steam is dry saturated steam and entrained water. Pressure is the means by which the high temperatures are obtained. Specific temperatures required to ensure microbicidal activity are 121°C (250°F) and 132°C (270°F). To ensure complete microbial destruction, these temperatures must be maintained for a specified minimal time.
There are two types of steam sterilizers (autoclaves): 1) gravity displacement, and 2) high-speed pre-vacuum. A gravity displacement autoclave works by pushing steam in through the top or sides of the sterilizing chamber and, because steam is lighter than air, forces the air out of the bottom of the chamber through a drain vent. This type of sterilizer requires longer cycles for porous materials because it is more difficult to remove air from porous materials. Pre-vacuum sterilizers use a vacuum pump / ejector to push air out of the sterilizing chamber before steam is pushed in. This type of sterilizer produces instant steam penetration into all loads, including porous materials.
The use of steam sterilizers requires quality testing. For pre-vacuum sterilizers, the Bowie-Dick test is used to detect air leaks and inadequate air removal. This test is required before the first processed load each day the sterilizer is used. If the sterilizer fails the Bowie-Dick test, the sterilizer is immediately removed from service until it is inspected by appropriate personnel and subsequently passes a Bowie-Dick test.
Other methods of monitoring the steam cycle include mechanical, chemical, and biological monitors. Each sterilizer should have a printout or graphic depiction of the temperature, the time maintained at temperature, and the pressure. These printouts should be evaluated by the sterile processing technician to ensure all parameters are met. The technician should sign the printout/graph with his/her full signature. Printouts are attached to the list of items included in the load and are usually stored for three years. Chemical indicators indicate the time and temperature parameters were met and are affixed to the outside or incorporated into the inside of the pack. Biological indicators (BI) monitor the effectiveness of steam sterilization daily when the sterilizers are in use. The BI contain spores of Geobacillis stearothermophilus; the test is conducted by using a control and a test container of the spores. The control and the test container are incubated and then evaluated for the presence or absence of spores. Positive spore test results are rare; however, the cause of failure must be investigated (operator error, inadequate steam delivery, or equipment malfunction). If the positive spore test is valid, then all items processed in that sterilizer since the last negative result must be recalled. The items are identified by the line listing of items from that sterilizer, retrieved from the end-user sterile storage room, and reprocessed.
Immediate Use Steam Sterilization (IUSS; formerly known as Flash Sterilization). IUSS is a modification of conventional steam sterilization in which the flashed item is placed in a specially designed, covered, rigid container to allow rapid penetration of steam. IUSS typically can be accomplished within three minutes and is generally used only in an operating room environment. This type of sterilization is not recommended because timely reports of biological indicators are not available, protective packaging is absent, the potential for contamination during transport to the operating room is a risk, and the sterilization cycle parameters (time, temperature, pressure) are minimal.
Ethylene oxide (EO). This type of low-temperature sterilization is useful for items that are temperature and moisture sensitive. The microbicidal activity of EO is accomplished by disruption of normal cellular metabolism and replication.
The four essential parameters associated with EO sterilization include: 1) gas concentration (450 to 1200 mg/ml), 2) temperature (37 – 63°C), 3) relative humidity (40-80%) because water molecules carry EO to reactive sites, and 4) exposure time (1-6 hours). EO can be used to sterilize medical devices that are heat or moisture sensitive without causing damage to the device. Disadvantages include the lengthy time cycle, cost, and potential hazards to patients and staff. After the 2-1/2 hour sterilization cycle, mechanical aeration for 8 to 12 hours is required to ensure desorption of the toxic EO residual contained in the exposed absorbent materials.
Hydrogen Peroxide Gas Plasma. Gas plasmas are generated in an enclosed chamber under deep vacuum using radio frequency or microwave energy to excite the gas molecules and produce charged particles in the form of free radicals. These free radicals interact with essential cell components (e.g., enzymes, nucleic acids) and disrupt the metabolism of the microorganism. The type of seed gas used and the depth of the vacuum determine effectiveness.
Because the by-products of the cycle (e.g., water vapor, oxygen) are nontoxic, the need for aeration is eliminated. This process is much quicker than EO, and ranges from 28-75 minutes. All items in the chamber must be dry. The presence of moisture prevents achievement of the vacuum and results in abortion of the cycle. The biological indicator used with hydrogen peroxide gas plasma is Bacillus atrophaeus spores.
Peracetic Acid Sterilization. This chemical is used in automated machines to sterilize medical, surgical, and dental instruments. Peracetic acid is an oxidizing agent that denatures proteins, disrupts cell wall pearmeability, and oxidizes sulfhydral and sulfur bonds in proteins, enzymes, and other metabolites. The biological monitor is Geobacillis stearothermophilus.
Exercise #8
Take a tour of your facility and for each of the listed sterilization methods describe where it is done in your facility and give an example(s) of a medical device it is used on: Steam Sterilization, Hydrogen peroxide gas plasma, Ethylene oxide (EO), Flash sterilization (IUSS), Chemical sterilant.
Event Related Sterility
Contamination of medical instruments within packaging is considered to be “event-related” as opposed to “time-related.” An event must occur to compromise the sterility of the medical equipment within packaging material. If the contamination was “time-related” an expiration date would be used. However, neither the Joint Commission on Accreditation of Healthcare Organizations nor the CDC require an expiration date on packaged medical equipment.
Current packaging materials are approved by the FDA and classified as Class II Medical Devices. These materials can include peel pouches, rigid containers and non-woven wraps. Contamination events may occur by:
- Poor barrier quality of the packaging materials (e.g., muslin)
- Microbial challenge (how dirty/soiled) is the environment?
- Events themselves: major events which compromise sterility include
- Microbes – type and quantity
- Location – where items are stored or transported
- Temperature – microbes thrive in temperatures around 98.6°F. Thus, the maximum temperature for the sterile storage area is 75°F.
- Air movement – some microbes travel in air currents. Stagnant air permits microbes to congregate. Thus, the recommended air movement in Sterile Storage is four (4) air exchanges per hour with positive pressure.
- Humidity – high humidity levels (above 70%) should be avoided. Packaging integrity may be adversely affected at high humidity levels.
- Traffic – the more people in the sterile storage area, the greater potential for contamination (turbulence).
- Opening the package – if aseptic technique is not used, package contents may become contaminated upon opening.
- Handling/transport – dropping or compressing the package.
- Visual evidence of compromise – packages must be inspected for tears or holes, rupture of the package seals, broken closures or missing locks from rigid containers, evidence of moisture (wetness) or crushed packages.3
Exercise #9
1. Define Sterilization.
2. For each of the listed sterilization methods, give a definition and list its advantages and disadvantages: Steam Sterilization, Hydrogen peroxide gas plasma, Ethylene oxide (EO), Chemical Sterilant, Immediate-use sterilization (IUSS; formerly known as flash sterilization), Event related sterility.
3. For each sterilization monitoring method indicator, give a definition and describe the type used: biological indicators, chemical indicators, physical indicators, Bowie Dick Test.
Manufacturers’ Recommendations for Reprocessing Medical Instruments and Equipment
According to the AORN, following manufacturers’ instructions for decontamination of instruments and equipment decreases the possibility of using cleaning agents that can damage the protective surfaces of instruments, contribute to corrosion, and impede sterilization.3 Instructions for handling and reprocessing should be obtained and evaluated by the IP prior to the purchase of the instrument or equipment. The facility must be able to adequately clean and reprocess the equipment. Sterile processing technicians should review manufacturers’ instructions before and during the reprocessing cycle.
A SPECIAL NOTE ABOUT Heating, Ventilation, Air Conditioning Systems in Central Sterile, Reprocessing, and Operating Rooms
Rooms in which contaminated instruments and endoscopes are reprocessed have specific requirements for heating and air conditioning (HVAC) systems. Rooms in which anesthesia gases are used must also meet strict HVAC requirements. Air changes per hour (ACH) are the number of times the air volume of a given space is replaced in a 1-hour period. The ACH should include sufficient changes of outside air to dilute microbial contamination and gases. Air movement is generally from clean to less clean areas. Positive air movement (air flow) means that air moves out from the room to the adjacent area. Negative air movement (air flow) means that air moves in from the adjacent area. Refer to table 13 for the ventilation requirements for reprocessing areas and operating rooms.
Table 13. HVAC requirements for reprocessing areas and operating rooms.
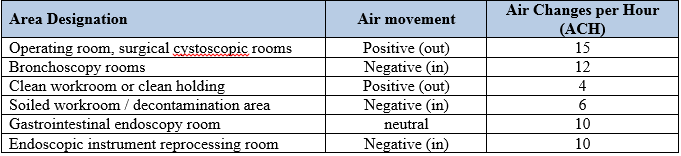
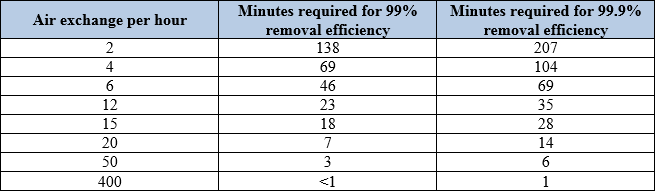
Ventilation systems in rooms where anesthesia gases are administered should have an air exchange rate of at least 15 exchanges per hour. The system should be operational at all times to maintain the air movement relationship to the adjacent areas. If the system is shut down for any length of time, the first surgical patient after the system is restarted may be exposed to contaminants and may contribute to a higher rate of surgical site infections. Refer to table 14 for a summary of the air exchanges per hour and the time required for airborne contaminant removal.
Table 14. Air exchanges rates.
Exercise #10
Meet with the facility engineering department HVAC staff member. Obtain data for the most recent assessment of the air flow and ACH in the areas listed in Table 15, as applicable for your facility. (Please see the printable PDF version of this section, linked at the top of the page, to view Table 15).
Do these results meet the requirements?
How often are these areas tested?
Request an annual report regarding air flow and ACH from the engineering department staff for the Infection Prevention and Control Committee.
Methods
Tours of Key Reprocessing Areas
The majority of the cleaning, disinfection and sterilization of medical devices is done in the Central Sterile Processing (CSP), which also may be called Central Sterile or Sterile Processing Services, the Operating Rooms (OR) including the c-section suite, Interventional Radiology, and Endoscopy. The IP should know the policies and procedures regarding environmental cleaning and disinfection, including:
- Identification of responsible personnel
- Competency validation
- Standard cleaning and disinfection procedures
- Frequency of cleaning and disinfection
- Chemicals approved by the Infection Prevention and Control Committee for use
- Labeling and secondary containers
- Required personal protective equipment (PPE) and the proper use of PPE
Exercise #11
Inspect reprocessing areas in your facility and spend time observing processes in each. Tables 16, 17, 18 and 19 contain a checklist you can use while touring these areas. (Please see the downloadable PDF version of this section to see Tables 16, 17, 18, and 19).
Prepare for your tour by reviewing these departments’ infection control programs, educational programs, roles and responsibilities of personnel in these departments, algorithms, etc.
Note the presence of dedicated hand hygiene sinks, hygiene practices, and supplies of personal protective equipment (PPE).
Exercise #12
Conduct observations of reprocessing areas and answer the following questions:
Transporting & Handling of Contaminated Medical Equipment/Devices
- How is contaminated equipment transported to the CSP department or reprocessing area
-
- Within the facility(department to department)
- Between facilities (vehicle transport, staff training certification for staff transporting of hazardous goods)
- Do the policies and procedures include information on transportation of contaminated equipment or medical devices?
- Do device transport methods meet the best practice standards?
- Do offsite clinics/facilities clean/reprocess their own medical devices/items?
- Where are sterile items stored in your facility? (exact locations)
Sterile Storage and Handling
- Where are sterile items stored in your facility? (exact locations)
- How are they stored?
- Dust barrier?
- Temp/humidity monitoring?
- Solid bottom shelf
- Is there evidence that stock is rotated? (FIFO – First In, First Out)
- Are items stacked? Only rigid containers should be stacked.
- Do storage areas have limited access?
- When was storage areas last inspected? Look for documentation.
- When was the last in-service given on sterile storage requirements? Look for documentation.
- How are sterile items transported?
-
- Covered / enclosed cart with a solid bottom shelf?
- Are reusable cart covers cleaned after each use?
- Are items stacked on one another during transport/distribution?
-
- Ask staff members what they do if a pack is dropped on the floor, compressed, torn, or wet.
Documentation and Reporting
Reports from all departments that conduct medical instrument and device reprocessing should be reviewed by the IP. Reports should be summarized and/or graphically depicted for presentation to the Infection Control Committee.
Table 22. IP Reports.

Resources
Helpful/Related Readings
- Bennett J and Brachman P, eds. Bennett & Brachman’s Hospital Infection 6th Edition. Philadelphia, PA: William R Jarvis; 2014.
- Chapter 20, Disinfection and Sterilization in Healthcare Facilities, by WA Rutala and DJ Weber
- Bennett G, Morrell G, and Green L, ed. Infection Prevention Manual for Hospitals; revised edition. Rome, GA: ICP Associates, Inc.; 2010.
- Section 6: pages 6-24, 39, 43
- Section 7: pages 4-7
- Bennett G. Infection Prevention Manual for Ambulatory Care. Rome, GA: ICP Associates Inc.; 2009.
- Section 6: pages 4-23, 41-42
- Section 7: pages 4-9.
- Bennett G and Kassai M. Infection Prevention Manual for Ambulatory Surgery Centers. Rome, GA: ICP Associates, Inc.; 2011. Section 8: pages 1-68.
- Lautenbach E, Woeltje KF, and Malani PN, ed. SHEA Practical Healthcare Epidemiology (3rd Edition). University of Chicago Press, Chicago, IL; 2010
- Chapter 7 Disinfection and Sterilization in Healthcare Facilities, by WA Rutala and DJ Weber
- Mayhall CG, ed. Hospital Epidemiology and Infection Control (4th Edition). Philadelphia, PA: Lippincott Williams & Wilkins, a Wolters Kluwer business; 2011.
- Chapter 70, Central Sterile Supply, by LM Sehulster
- Chapter 80, Selection and Use of Disinfectants in Healthcare, by WA Rutala and DJ Weber
- Chapter 81, Sterilization and Pasteurization in Healthcare Facilities, by LM Sehulster and WW Bond
- Chapter 84, Design and Maintenance of Hospital Ventilation Systems and the Prevention of Airborne Healthcare-Associated Infections, by AJ Streifel
Helpful Contacts (in WY or US)
- Baerbel Merrill, MS, BSN, CIC, michbaer@bresnan.net, 1-307-689-3942
- Deborah F. Wilson, MS, RN, CIC, deborah.wilson5@va.gov, 307-778-7550, ext. 7091
Related Websites/Organizations
- Wyoming Department of Health, Infectious Disease Epidemiology Unit, Healthcare-Associated Infection Prevention: health.wyo.gov/phsd/epiid/HAIgeneral.html
- Mountain-Pacific Quality Health – Wyoming: mpqhf.com/wyoming/index.php
- Occupational Safety and Health Administration (OSHA): osha.gov/
- Centers for Medicare and Medicaid Services (CMS): cms.gov/
- The Joint Commission (TJC): jointcommission.org/
- American College of Surgeons (ACS): facs.org/
- American Society of Anesthesiologist (ASA): asahq.org/
- Association of periOperative Registered Nurses (AORN): aorn.org/
- S. Food and Drug Administration (FDA): www.fda.gov/
- S. Environmental Protection Agency (EPA): www.epa.gov/
- Association for the Advancement of Medical Instrumentation (AAMI): aami.org/
- Association for Professionals in Infection Control and Epidemiology (APIC): apic.org/
- STERIS University, a place to gain online Continuing Education Units specifically related to device reprocessing, infection prevention and control and surgical care: steris.com/sterisu/
References
- Henry Schein Medical. Available at: henryschein.com/us-en/medical/resourcecenter/instrumentreprocessdecontaminationlifecycle.aspx . Accessed February 25, 2014.
- Rutala, W., et al. Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. 2009. Available at: cdc.gov/hicpac/pdf/guidelines/Disinfection_Nov_2008.pdf Accessed March 3, 2014.
- Association of peri-Operative Registered Nurses, Perioperative Standards and Recommended Practices, Recommended Practices for Cleaning and Care of Surgical Instruments and Powered Equipment. Available at: aornstandards.org/content/1/SEC40.body. Accessed March 7, 2014.

WIPAG welcomes your comments and feedback on these sections.
For comments or inquiries, please contact:
Cody Loveland, MPH, Healthcare-Associated Infection (HAI) Prevention Coordinator
Infectious Disease Epidemiology Unit,
Public Health Sciences Section, Public Health Division
Wyoming Department of Health
6101 Yellowstone Road, Suite #510
Cheyenne, WY 82002
Tel: 307-777-8634 Fax: 307-777-5573
Email: cody.loveland@wyo.gov

